Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 Sr
Sr CoO
CoO at initial electrochemical process in an alkaline solution
at initial electrochemical process in an alkaline solutionMatsuzaki, Akira*; Hirayama, Masaaki*; Oguchi, Shoya*; Komo, Mamoru*; Ikezawa, Atsunori*; Suzuki, Kota*; Tamura, Kazuhisa; Arai, Hajime*; Kanno, Ryoji*
Electrochemistry (Internet), 90(10), p.107001_1 - 107001_8, 2022/10
Times Cited Count:0 Percentile:0.01(Electrochemistry)Oxygen reduction and evolution reactions (ORR and OER) of perovskite-type La Sr
Sr CoO
CoO were characterized using two-dimensional model electrodes with different reaction planes. Synthesized by pulsed laser deposition, these thin and flat electrodes can reveal the reaction plane dependence of the ORR activity. From steady-state polarization measurements in KOH (aq.), the ORR activity was the highest on the (001) film during the first ORR/OER cycle, and it decreased significantly during the second cycle. In-situ synchrotron X-ray diffraction clarified crystal structure changes in the bulk and surface regions of La
were characterized using two-dimensional model electrodes with different reaction planes. Synthesized by pulsed laser deposition, these thin and flat electrodes can reveal the reaction plane dependence of the ORR activity. From steady-state polarization measurements in KOH (aq.), the ORR activity was the highest on the (001) film during the first ORR/OER cycle, and it decreased significantly during the second cycle. In-situ synchrotron X-ray diffraction clarified crystal structure changes in the bulk and surface regions of La Sr
Sr CoO
CoO , and these changes are associated with forming oxygen defects during the initial electrochemical process. Furthermore, the La
, and these changes are associated with forming oxygen defects during the initial electrochemical process. Furthermore, the La Sr
Sr CoO
CoO surface partially decomposed upon reacting. Therefore, the interfacial structures formed in the electrochemical reaction field is important for enhancing ORR and OER activities.
surface partially decomposed upon reacting. Therefore, the interfacial structures formed in the electrochemical reaction field is important for enhancing ORR and OER activities.
Higuchi, Yuki*; Setoyama, Daigo*; Isegawa, Kazuhisa; Tsuchikawa, Yusuke; Matsumoto, Yoshihiro*; Parker, J. D.*; Shinohara, Takenao; Nagai, Yasutaka*
Physical Chemistry Chemical Physics, 23(2), p.1062 - 1071, 2021/01
Times Cited Count:6 Percentile:50.99(Chemistry, Physical)This study is the first report on liquid water and ice imaging conducted at a pulsed spallation neutron source facility. Neutron imaging can be utilised to visualise the water distribution inside polymer electrolyte fuel cells (PEFCs). Particularly, energy-resolved neutron imaging is a methodology capable of distinguishing between liquid water and ice, and is effective for investigating ice formation in PEFCs operating in a subfreezing environment. The distinction principle is based on the fact that the cross sections of liquid water and ice differ from each other at low neutron energies. In order to quantitatively observe transient freezing and thawing phenomena in a multiphase mixture (gas/liquid/solid) within real PEFCs with high spatial resolution, a pulsed neutron beam with both high intensity and wide energy range is most appropriate. In the validation study of the present work, we used water sealed in narrow capillary tubes to simulate the flow channels of a PEFC, and a pulsed neutron beam was applied to distinguish ice, liquid water and super-cooled water, and to clarify freezing and thawing phenomena of the water within the capillary tubes. Moreover, we have enabled the observation of liquid water/ice distributions in a large field of view (300 mm  300 mm) by manufacturing a sub-zero environment chamber that can be cooled down to -30
300 mm) by manufacturing a sub-zero environment chamber that can be cooled down to -30 C, as a step towards
C, as a step towards  visualisation of full-size fuel cells.
visualisation of full-size fuel cells.
Kishi, Hirofumi*; Sakamoto, Tomokazu*; Asazawa, Koichiro*; Yamaguchi, Susumu*; Kato, Takeshi*; Zulevi, B.*; Serov, A.*; Artyushkova, K.*; Atanassov, P.*; Matsumura, Daiju; et al.
Nanomaterials (Internet), 8(12), p.965_1 - 965_13, 2018/12
Times Cited Count:11 Percentile:48.71(Chemistry, Multidisciplinary)Cui, Y.-T.*; Harada, Yoshihisa*; Niwa, Hideharu*; Oshima, Masaharu*; Hatanaka, Tatsuya*; Nakamura, Naoki*; Ando, Masaki*; Yoshida, Toshihiko*; Ishii, Kenji*; Matsumura, Daiju
NanotechJapan Bulletin (Internet), 11(4), 6 Pages, 2018/08
no abstracts in English
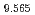 (Si
(Si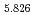 []
[]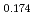 )O
)O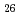 apatite without interstitial oxygens due to the overbonded channel oxygens
apatite without interstitial oxygens due to the overbonded channel oxygensFujii, Kotaro*; Yashima, Masatomo*; Hibino, Keisuke*; Shiraiwa, Masahiro*; Fukuda, Koichiro*; Nakayama, Susumu*; Ishizawa, Nobuo*; Hanashima, Takayasu*; Ohara, Takashi
Journal of Materials Chemistry A, 6(23), p.10835 - 10846, 2018/06
Times Cited Count:27 Percentile:69.76(Chemistry, Physical) O
O /C hydrazine electrooxidation catalysts for anion exchange membrane fuel cells
/C hydrazine electrooxidation catalysts for anion exchange membrane fuel cellsSakamoto, Tomokazu*; Masuda, Teruyuki*; Yoshimoto, Koji*; Kishi, Hirofumi*; Yamaguchi, Susumu*; Matsumura, Daiju; Tamura, Kazuhisa; Hori, Akihiro*; Horiuchi, Yosuke*; Serov, A.*; et al.
Journal of the Electrochemical Society, 164(4), p.F229 - F234, 2017/01
Times Cited Count:13 Percentile:43.11(Electrochemistry)Sakamoto, Tomokazu*; Kishi, Hirofumi*; Yamaguchi, Susumu*; Matsumura, Daiju; Tamura, Kazuhisa; Hori, Akihiro*; Horiuchi, Yosuke*; Serov, A.*; Artyushkova, K.*; Atanassov, P.*; et al.
Journal of the Electrochemical Society, 163(10), p.H951 - H957, 2016/08
Times Cited Count:30 Percentile:76.17(Electrochemistry)Sakamoto, Tomokazu*; Kishi, Hirofumi*; Yamaguchi, Susumu*; Tanaka, Hirohisa*; Matsumura, Daiju; Tamura, Kazuhisa; Nishihata, Yasuo
Hyomen Kagaku, 37(2), p.78 - 83, 2016/02
We have developed direct liquid fuel anion exchange membrane fuel cell vehicles to deal with the global warming. Non-platinum group metals (PGM) catalyst has been researched to apply for both anode and cathode electrodes. A test driving was carried out for the fuel cell vehicle equipped with no precious metals as catalysts at SPring-8 in 2013. Here we introduce our results of advanced analysis for reaction mechanism and active site of non-PGM catalyst using synchrotron radiation X-rays at SPring-8.
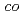 -tetrafluoroethylene) films using different media
-tetrafluoroethylene) films using different mediaNuryanthi, N.*; Yamaki, Tetsuya; Kitamura, Akane; Koshikawa, Hiroshi; Yoshimura, Kimio; Sawada, Shinichi; Hasegawa, Shin; Asano, Masaharu; Maekawa, Yasunari; Suzuki, Akihiro*; et al.
Transactions of the Materials Research Society of Japan, 40(4), p.359 - 362, 2015/12
The ion-track grafting of a vinylbenzyl chloride (VBC) into a poly(ethylene-co-tetrafluoroethylene) (ETFE) film is necessary for preparing nanostructured hydroxide-ion-conductive electrolyte membranes. A key for success here is to obtain as high graft levels as possible (for higher conductivity) in a smaller number of tracks (for improving the other membrane properties). To this end, therefore, the effect of the medium for the VBC grafting was investigated as part of our continuing effort to optimize the experimental conditions. A 25  m-thick ETFE film was irradiated in a vacuum chamber with 560 MeV
m-thick ETFE film was irradiated in a vacuum chamber with 560 MeV  Xe at different fluences, and then the grafting was performed by immersing the irradiated films in a 20vol% VBC monomer at 60
Xe at different fluences, and then the grafting was performed by immersing the irradiated films in a 20vol% VBC monomer at 60 C. A medium was a mixture of water (H
C. A medium was a mixture of water (H O) and isopropyl alcohol (iPrOH) at different volume ratios. The degree of grafting increased as the H
O) and isopropyl alcohol (iPrOH) at different volume ratios. The degree of grafting increased as the H O content became higher, and reached a maximum in pure H
O content became higher, and reached a maximum in pure H O. These results can be explained by considering the well-known Trommsdorff effect, in which poor solubility of the grafted polymer in polar media leads to an increased polymerization rate probably due to a lower termination rate.
O. These results can be explained by considering the well-known Trommsdorff effect, in which poor solubility of the grafted polymer in polar media leads to an increased polymerization rate probably due to a lower termination rate.
Sakamoto, Tomokazu*; Matsumura, Daiju; Asazawa, Koichiro*; Martinez, U.*; Serov, A.*; Artyushkova, K.*; Atanassov, P.*; Tamura, Kazuhisa; Nishihata, Yasuo; Tanaka, Hirohisa*
Electrochimica Acta, 163, p.116 - 122, 2015/05
Times Cited Count:57 Percentile:83.48(Electrochemistry)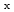 promoter on Pt electro-catalyst
promoter on Pt electro-catalystFugane, Keisuke*; Mori, Toshiyuki*; Yan, P.*; Masuda, Takuya*; Yamamoto, Shunya; Ye, F.*; Yoshikawa, Hideki*; Auchterlonie, G.*; Drennan, J.*
ACS Applied Materials & Interfaces, 7(4), p.2698 - 2707, 2015/02
Times Cited Count:30 Percentile:66.8(Nanoscience & Nanotechnology)no abstracts in English
Asazawa, Koichiro*; Kishi, Hirofumi*; Tanaka, Hirohisa*; Matsumura, Daiju; Tamura, Kazuhisa; Nishihata, Yasuo; Saputro, A. G.*; Nakanishi, Hiroshi*; Kasai, Hideaki*; Artyushkova, K.*; et al.
Journal of Physical Chemistry C, 118(44), p.25480 - 25486, 2014/11
Times Cited Count:15 Percentile:44.72(Chemistry, Physical)Yamaki, Tetsuya; Asano, Masaharu; Yoshida, Masaru
Gekkan Eko Indasutori, 10(6), p.5 - 11, 2005/06
no abstracts in English
Yamaki, Tetsuya; Asano, Masaharu; Yoshida, Masaru
Kogyo Zairyo, 53(1), p.63 - 67, 2005/01
no abstracts in English
Yamaki, Tetsuya; Yoshida, Masaru
Nenryo Denchi, 4(3), p.73 - 78, 2005/01
no abstracts in English
Yamaki, Tetsuya; Asano, Masaharu; Maekawa, Yasunari; Morita, Yosuke; Suwa, Takeshi; Chen, J.*; Tsubokawa, Norio*; Kobayashi, Kazuhiro*; Kubota, Hitoshi*; Yoshida, Masaru
Radiation Physics and Chemistry, 67(3-4), p.403 - 407, 2003/08
Times Cited Count:76 Percentile:97.01(Chemistry, Physical)no abstracts in English
Yamaki, Tetsuya; Asano, Masaharu; Morita, Yosuke*; Suwa, Takeshi*; Yoshida, Masaru
Proceedings of 9th International Conference on Radiation Curing (RadTech Asia '03) (CD-ROM), 4 Pages, 2003/00
Proton exchange membranes were prepared by the radiation-induced grafting of styrene into crosslinked polytetrafluoroethylene (PTFE) films and subsequent sulfonation. The degree of grafting was controlled in the range of 7-75% by the crosslinking density of the PTFE matrix as well as the grafting conditions. The films at the grafting yield of  30% were found to give the ion exchange membranes with a homogeneous distribution of sulfonic acid groups. The resulting membranes showed a large ion exchange capacity reaching 2.9 meq g
30% were found to give the ion exchange membranes with a homogeneous distribution of sulfonic acid groups. The resulting membranes showed a large ion exchange capacity reaching 2.9 meq g , which exceeded the performance of commercially-available perfluorosulfonic acid films such as Nafion. This will encourage the use of our ion exchange membranes in polymer electrolyte fuel cells.
, which exceeded the performance of commercially-available perfluorosulfonic acid films such as Nafion. This will encourage the use of our ion exchange membranes in polymer electrolyte fuel cells.
Suwa, Takeshi; Morita, Yosuke
Hoshasen To Sangyo, (93), p.22 - 28, 2002/03
no abstracts in English
Suwa, Takeshi
Porima Daijesuto, 54(3), p.17 - 26, 2002/03
no abstracts in English
Kohara, Shinji*; Suzuya, Kentaro; Ono, Hideo
Proceedings of 12th International Symposium on Molten Salts (Molten Salts 12), 99-41, p.253 - 262, 1999/10
no abstracts in English